The Role of Immunotherapy and the Management of Asthma: Systematic Review
Background and Objectives for the Systematic Review
Rationale for the current systematic review
In 1989, the National Heart, Lung and Blood Institutes (NHLBI) initiated the National Asthma Education and Prevention Program (NAEPP) to address growing concern about asthma in the US. One of the first accomplishments of the NAEPP was to convene a panel of experts who summarized their recommendations in a document, National Asthma Education and Prevention Program Expert Panel Report (EPR): Guidelines for the Diagnosis and Management of Asthma, in 1991. The guidelines address the diagnosis, evaluation, and treatment of asthma. Given the most recent report, EPR-3, was published in 20071, NHLBI assessed the need for an update by requesting information from the public, NAEPP Coordinating Committee Members and its affiliates, and members of the 2007 Expert Panel. Collected information was provided to the NHLBI Advisory Council Asthma Expert Working Group, which produced a report to summarize the process and recommendations from their needs assessment2. The Working Group identified six high priority topics that should be updated. For each topic, key questions meriting a systematic literature review were formulated. NHLBI engaged AHRQ to perform the systematic reviews through its Evidence-based Practice Centers (EPC). This systematic review focuses on one of the high priority topics, allergen specific immunotherapy (AIT) for the treatment of asthma, which will be used to update EPR-3. The review also will highlight areas of controversy and identify needs for future research on this priority area.
Burden of asthma
Asthma is a chronic inflammatory disorder of the airways, characterized by varying degrees of airflow obstruction. Bronchoconstriction, inflammatory cell infiltration, and airway edema reduce airflow intermittently, often in response to specific exposures, resulting in respiratory symptoms.1 In the United States (US), the current prevalence of asthma has increased over the past decade, from an estimated 22.2 million Americans in 2005 to 24.0 million Americans in 2014.3,4Asthma can significantly impact patients' and families' quality-of-life and ability to pursue activities such as school, work, and exercise. Globally, asthma ranks 14th based on the burden of disease, as measured by disability adjusted life years.5 In the US, asthma contributes significantly to healthcare resource utilization and associated costs. For example, in 2012, asthma was one of the top twenty leading diagnosis groups for primary care visits and was the main reason for 1.8 million emergency department visits and 439,000 hospitalizations. While the severity of disease varies between patients and over time in the same patient, asthma can be fatal, accounting for approximately 1 death per 100,000 Americans.6
Allergic asthma
Asthma is a chronic lung disease that inflames and narrows the airways and causes recurring periods of wheezing, chest tightness, shortness of breath, and coughing. This disease affects people of all ages, but it most often starts during childhood. In the United States, more than 24 million people are known to have asthma. About 8.6% of these people are children.3 Approximately 62% percent of individuals with asthma also have allergies, as evidenced by atopy (i.e., one or more positive specific IgE). Allergic asthma and non-allergic asthma generally have the same symptoms; however, allergic asthma is triggered by inhaling allergens. An allergen is a typically harmless substance such as dust mites, pet dander, pollen or mold. Allergens trigger a response starting in the immune system and through a complex reaction, these allergens then cause the passages in the airways of the lungs to become inflamed and swollen. In the United States, 78% of asthmatic children are skin test positive for one or more inhalant allergens, and 75% of middle aged adult asthmatics.7 EPR-3 recommends that immunotherapy may be used "when there is clear evidence of a relationship between symptoms and exposure to an allergen to which the patient is sensitive (Evidence B)."
Description of the intervention – Allergen-specific immunotherapy
Immunotherapy, as a treatment for allergic diseases, was first introduced by Noon and Freeman in 1911 as a means of treating grass-induced allergic symptomatology – "hay fever" (i.e., rhinoconjunctivitis).8 In the United States (U.S.), individuals who are allergic to specific exposures (allergens) may receive escalating doses of a solution which contains extracts of the relevant allergen(s). The amount of allergen is initially very small and then escalated with the expectation that the individual's immune system will become more tolerant to the offending allergen with repeated exposures9,10 Controlled clinical trials have demonstrated therapeutic efficacy in controlling allergic symptoms including asthma, and have detailed the favorable immunologic changes associated with AIT for the treatment of allergic rhinitis, and asthma.11,12
One form of AIT, conventional subcutaneous immunotherapy (SCIT), involves injections of the allergen containing solution into the skin with, administration once or twice weekly. When the individual reaches a predetermined "maintenance" dose (for inhalant allergens, a range of 5-20 µg of major allergen), the frequency of dosing is reduced. The optimal duration of SCIT with an FDA approved allergen extract is not well defined, though, the maintenance dose is typically given once or twice monthly for 3 to 5 years.13 SCIT is efficacious in the treatment of allergic rhinitis, allergic conjunctivitis, allergic asthma, and stinging insect hypersensitivity, in adults and children.14,15 In addition, the effects are frequently long-term, and SCIT may prevent the development of asthma in patients with allergic rhinitis.16However, SCIT is ineffective in some patients, and although rare, there is a potential risk for serious systemic allergic reactions.14,17-19 In addition, patients may have poor compliance with SCIT due to the inconvenience of needing to travel to a physician's office for injections, and adverse reactions to injections. Standards of care indicate that patients should receive SCIT injections under the supervision of their provider in a facility with the appropriate equipment, medications and personnel to treat anaphylaxis, and be monitored for systemic reactions for 30 minutes following the injection.
Through the years, various chemical modifications of allergens have been attempted to enhance efficacy, improve safety, and foster compliance with AIT. Many of these previous approaches have been unsuccessful – in that the allergenicity (potential to cause an untoward allergic reaction) and immunogenicity (potential to induce a beneficial clinical effect) have either decreased, or increased in tandem, with no resultant efficacy/safety benefit ratio; however, recent approaches with modified allergens, adjuvants, including immunostimulatory adjuvants, recombinant allergens, T-cell tolerizing constructs, and improved oral approaches have been demonstrated in various clinical studies to be successful agents for treatment of allergic respiratory disease.20-22
Other routes of administration for AIT have been assessed, including sublingual immunotherapy (SLIT), which comes in both aqueous and tablet formulations. For this report, both sublingual aqueous and sublingual tablet formulations will be included. The rationale for this route of therapy is based on its perceived improved safety margin (reduced risk of anaphylaxis), simple and convenient oral dosing regimen (avoiding the discomfort of injections and the inconvenience of office visits for allergy shots), and possibly a decreased time to achieve effect.
Allergen-specific immunotherapy for allergic asthma
Although there is strong evidence for AIT's effectiveness in the treatment of allergic rhinoconjunctivitis, the available evidence supporting its efficacy for the treatment of allergic asthma, particularly its comparative effectiveness relative to available pharmacotherapy, is less robust.23 A 2010 Cochrane review showed that AIT produced a significant reduction in asthma symptoms and medication use, and improvement in nonspecific bronchial hyperreactivity as measured by response to methacholine or acetylcholine challenge tests.24 The 2011 Practice Parameters by the Joint Task Force (comprised of members from the American Academy of Allergy, Asthma & Immunology, the American College of Allergy, Asthma and Immunology and the Joint Council on Allergy, Asthma and Immunology) concluded noted that certain patients with allergic asthma may be indicated for AIT: "Candidates for immunotherapy are patients whose symptoms are not controlled adequately by medications and avoidance measures or those experiencing unacceptable adverse effects of medications or who wish to reduce the long-term use of medications."25 In 2013, the Johns Hopkins University Evidence-based Practice Center (JHU EPC), commissioned by AHRQ, completed a review of AIT for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma.26This review found high strength of evidence that SCIT can be effective in reducing asthma symptoms and medication use and SLIT in the aqueous form reduces asthma symptoms. Use of AIT in specific settings, and different forms of AIT will be considered in this systematic review, as well as SLIT in the tablet form.
Objectives
The current review focuses on the efficacy and safety of AIT for allergic asthma. The scope includes updating the data on the efficacy of SCIT since the 2013 AHRQ JHU EPC review,26 as well as considering new questions on the safety of SCIT administration and the efficacy and safety of SLIT. Concomitantly, this review will address several questions that remain unanswered about the use of AIT, including:
- Optimal dose, dosing frequency of allergen administration, and duration of treatment for AIT
- Compliance with AIT
- Efficacy and safety of AIT among subgroups of interest
Key Questions
- What is the evidence for the efficacy of Subcutaneous Immunotherapy (SCIT) in the treatment of asthma?
- Does this vary among subpopulations of interest?
- Does this vary by setting?
- Clinic
- Home
- What is the evidence for the safety of Subcutaneous Immunotherapy (SCIT) in the treatment of asthma?
- Does this vary among subpopulations of interest?
- Does this vary by setting?
- Clinic
- Home
- What is the evidence for the efficacy of Sublingual Immunotherapy (SLIT), in tablet and aqueous form, for the treatment of asthma?
- Does this vary among subpopulations of interest?
- Does this vary by setting?
- Clinic
- Home
- What is the evidence for safety of SLIT, in tablet and aqueous form, for the treatment of asthma?
- Does this vary among subpopulations of interest?
- Does this vary by setting?
- Clinic
- Home
The efficacy (KQ1) and safety (KQ2) of subcutaneous immunotherapy (SCIT) administered in the clinic and home, for asthma treatment.
The 2013 AHRQ JHU EPC report26 on AIT covered few measures of efficacy, effectiveness, and safety of SCIT administered in a clinical setting for asthma. The EPR-3 recommendations stipulate that immunotherapy should only be administered in a physician's office, where facilities and trained personnel are available to treat any reactions that could occur, but it is known that home administration does occur. Little has been published about the safety of home immunotherapy, but a 1999 study of home immunotherapy found the rate of major systemic reactions to be 0.005%.27 The 2013 AHRQ JHU EPC report did not specifically evaluate the safety of SCIT administered in the home, so the current report will evaluate the safety of home administration (KQ2), looking at the evidence starting one year before the search date ended for the EPR-3 review. SCIT efficacy in the clinic or home setting will be evaluated separately, since it may be different in these two settings. For this question (KQ1), the evidence review will start one year prior to the end search date of the AHRQ JHU EPC 2013 report evidence review (December 2012).
The efficacy (KQ 3) and safety (KQ 4) of sublingual immunotherapy (SLIT), in tablet and aqueous form, administered in the clinic and home, for asthma treatment
Although the AHRQ report26 reviewed the safety and efficacy of SLIT, it did not include the tablet form of SLIT, since no tablets had been approved by FDA at the time of that review. Three allergen extracts sublingual tablets for grasses28,29 and ragweed30 have been approved by the FDA31 since the 2013 AHRQ JHU EPC evidence review, and this review will evaluate the evidence of the efficacy (KQ 3) and safety (KQ 4) of these tablets and update the previous review of the aqueous form.
Population(s)
We will include studies enrolling patients of any ages with allergic asthma.
- Ideally studies will include patients with allergic asthma whose symptoms are not controlled adequately by medications and allergen avoidance measures or those experiencing unacceptable adverse effects of medications or who wish to reduce the long-term use of medications.32 However, many studies may not define clearly the population or characterize indicators of symptom control, which may limit the accuracy of the population definition.
- Patients with diagnosis of asthma and positive allergy testing based on allergen specific IgE sensitization diagnosis: Serologic multiallergen screen IgE tests (skin prick tests, serum tests, or both)
- Patients with all severity grades and control status of asthma (based on the EPR-3 classification).1
- Studies with a population that is not limited to those with asthma (e.g., includes patients with allergic rhinitis and asthma) will be included only if the outcomes for asthma patients are presented separately (i.e., stratified).
Subgroups of interest include:
- Receipt of single or multi-allergen therapy
- Pediatric or adult populations
- For studies with pediatric patients, we will present results, where possible, by the categories: 0-4 years old, 5-11 years old and older than 12 years old1
- Grouping by asthma severity and asthma control
- Grouping by patients with documented asthma symptoms on exposure to allergen and those without
Interventions
KQ1 and KQ2
Subcutaneous immunotherapy
KQ3 and KQ4
Sublingual immunotherapy, tablet form or aqueous form
All KQs
Dosing and schedule (build-up dosing and schedule, maintenance dosing and schedule, and duration of therapy)
Co-intervention
All KQs
Usual asthma care per EPR-31 (see Appendix 1)
Comparators
All KQs
- Usual care only
- Placebo
- SCIT vs SLIT
- Home vs Clinic administration
Outcomes
Outcomes for Key Questions 1 and 3
Asthma Outcomes (Based on the 2011 National Institute of Health (NIH) Asthma Outcomes workshop)33
- Asthma symptoms/outcomes
- Asthma control composite scores (for patients 12 years and older)
- Asthma Control Test (ACT)
- Asthma Control Questionnaire (ACQ)
- Pediatric Asthma Control Test (PACQ)
- Death (all cause and asthma related)
- Asthma control composite scores (for patients 12 years and older)
- Quality of Life
- Asthma-specific quality of life- Asthma Quality of Life Questionnaire (AQLQ)
- Pediatric Asthma-specific quality of life- Asthma Quality of Life Questionnaire (PAQLQ)
- School/Work absences
- Medication use
- Asthma specific medication use (name, dose, duration)
- Long-term controller medication use
- Quick relief medication use
- Systemic corticosteroids for asthma
- Asthma exacerbations / Healthcare utilization
- Asthma-specific hospitalizations
- Asthma-specific Emergency Department (ED) visits (separate urgent care visits when they can be differentiated)
- Asthma-specific ICU admission/intubations
- Asthma-specific outpatient visits
- Resource use related to the intervention (personnel time and equipment)
- Pulmonary physiology:
- Spirometry: Prebronchodilator and post bronchodilator (Peak expiratory flow (PEF) monitoring and lung volumes, forced expiratory volume (FEV), forced vital capacity (FVC), forced expiratory flow (FEF) as absolute, percent predicted and important ratios (FEV1/FVC) that reflect airway flow.
- Airway responsiveness (Methacholine inhalation and exercise challenge)
- Compliance with immunotherapy
Intermediate outcomes (KQ1 and KQ3)
- Immunologic parameters
- Allergy skin testing
- Allergen-specific Immunoglobulin E (IgE)
- Allergen-specific Immunoglobulin G4 (IgG4)
Outcomes for Key Questions 2 and 4
- Harms (Adverse events due to immunotherapy)
- Serious adverse events
- Anaphylaxis reaction
- Hypersensitivity reaction
- Other adverse effects of immunotherapy (local and systemic effects)
- Death (all-cause, asthma related)
Timing
All KQs
Studies with all lengths of follow-up duration will be considered.
Settings
All KQs
- Clinical setting
- Home setting
Study type
KQ1 and KQ3
- Included: RCTs
- Exclude: cohort studies, case reports, cross-sectional studies and surveys
KQ2 and KQ4
- Included: RCTs, cohort studies, case reports
- Excluded: Cross-sectional studies, surveys
Analytic Framework
Figure 1. Analytic framework for the role of allergen-specific immunotherapy in the treatment of asthma.
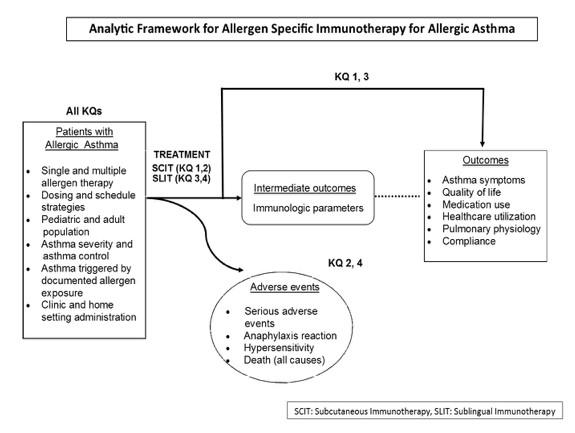
Figure 1. This figure depicts the key questions (KQs) within the context of the PICOTS and subgroups described in the previous section. In general, the figure illustrates how immunotherapy administered to patients with allergic asthma may result in intermediate outcomes such as changes in immunologic parameters and/or outcomes such as improvement of symptoms and quality of life and reduction of medication use. In addition, adverse events may occur at any point after treatment is received.
Methods
Criteria for Inclusion/Exclusion of Studies in the Review
For all the Key Questions, we will include randomized controlled trials (RCTs). For Key Questions 2 and 4 we will also include cohort studies and case reports to assess safety. For all the Key Questions we will exclude cross-sectional studies and surveys. We will also exclude cross-over studies because AIT is a long term treatment and no wash out period would be long enough to diminish carry-over. Our search will not be limited by language.
Searching for the Evidence: Literature Search Strategies for Identifying Relevant Studies to Answer the Key Questions
The 2013 JHU EPC review on "Allergen-specific immunotherapy for the treatment of allergic rhinoconjunctivitis and/or asthma" included SCIT and SLIT but excluded the tablet form of SLIT as it was not approved by the FDA at the time of the review. We will update the prior report (All KQs), adding the tablet form of SLIT (KQ3 and KQ4), as well as looking at the safety of AIT administered in the home (KQ2, KQ4). While the prior report search date was 2012, because of the change in scope, we will develop a broader search and search starting one year prior to the search date for the EPR-3 review (i.e., search date was 2006 so our search will start from 2005). The searches will be updated during the peer review process.
We will search PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL). The search strategy, in PubMed format is: (immunotherapy[mesh] OR immunotherap*[tiab]) AND (asthma[mh] OR asthma[tiab]) NOT ("occupational diseases" [mh]) NOT (animals[mh] NOT humans[mh])
Grey literature searches will be completed to identify data and studies not reported in the published literature, to assess for publication and reporting bias and to inform future research needs. Studies identified through grey literature searches will be considered for inclusion into the review under two conditions: 1) if they are a source of a unique study that meets inclusion criteria and provides enough methodologic detail to assess risk of bias or 2) if they can be matched to an original publication that has been included into the review when the abstract or presentation reports data on an outcome that was not provided by the original publication. We will handsearch the reference lists of all newly identified articles. Additionally, the team will search ClinicalTrials.gov to identify relevant registered trials and we will review any Scientific Information Packets provided by the manufacturers. We will also review the FDA Adverse Event Reporting System (FAERS).
Data Abstraction and Management
Potentially relevant citations will be screened by using DistillerSR (Evidence Partners Incorporated, Ottawa, Canada), a Web-based systematic review software. Citations identified by the search strategies will be uploaded to DistillerSR and managed in the following manner: Two reviewers will independently assess abstracts, and then full-text articles, resulting from the literature searches according to the inclusion criteria stated in Section IV. Any disagreements regarding inclusion will be resolved through discussion, and unresolved conflicts will be adjudicated during a team meeting of investigators and advisors. Studies labeled as "exclude" by both review authors will be excluded from the review and the reasons for exclusion documented.
Each article included will undergo data abstraction by two team members. A first reviewer will extract descriptions of the study methods to include the population, intervention(s), comparator[s], and outcomes of interest by using a form designed by the team, and the second reviewer will confirm the first reviewer's abstracted data for completeness and accuracy. Reviewer pairs will be formed to include personnel with both clinical and methodological expertise. A third reviewer will audit a random sample of articles to ensure consistency in the data abstraction of the articles.
We will collect data on subgroups of interest, including gender, age, ethnicity, asthma definition, asthma status and severity, immunotherapy details such as single, multiple, seasonal and perennial allergens and the dosing information. We will also collect information on cointerventions and rescue medications. We will collect information on study compliance/adherence and number of subjects that did not complete the study. We will include outcomes of interest as defined in our PICOTS by using a form designed by the team investigators and advisors.
Assessment of Risk of Bias of Individual Studies
We will use the Cochrane Collaboration's tool for assessing the risk of bias of randomized and quasi-randomized trials or an adaptation appropriate for our body of literature. Two authors will independently assess the included studies for sources of systematic bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions.34 We will use the Cochrane ROBINS-I tool to assess the methodological quality of non-randomized studies included.35 We will evaluate the studies for the following criteria: sequence generation and allocation concealment (selection bias), masking of participants, study investigators, outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias.
Judgments for each criterion will be reported as "Yes" (low risk of bias), "No" (high risk of bias), or "Unclear" (information is insufficient to assess) for RCTs and "Low" , "Moderate", "Serious", "Critical" or "no-info" for non RCTs. Two reviewers will conduct risk of bias assessment and resolve disagreements through discussion. We will contact the authors of the studies for additional information on issues that were unclear from information available in the original reports. In case of failure to communicate with the primary investigators, or if there is no response within 6 weeks, we will assess the methodological quality on the basis of the available information.
We will apply the World Health Organization (WHO) criteria to the case reports to judge the likelihood that the intervention was causally related to the observed serious adverse event.36
Data Synthesis
We will complete a qualitative synthesis for all questions. We will conduct meta-analyses when there are sufficient data and studies are sufficiently homogenous with respect to key variables (population characteristics, study duration, and treatment characteristics). If the I2 statistic suggests considerable heterogeneity (equal to or greater than 75%) or if there are insufficient data (less than three studies), we will not combine the results in a meta-analysis and will instead present a narrative summary. We will compute effect sizes and measures of variance using standard methods and will perform random-effects meta-analysis using the Hartung-Knapp method.37
We will consider preparing funnel plots in pooled analyses with more than 10 studies to assess the presence of reporting biases in conjunction with study characteristics or other factors that may contribute to asymmetry of the plot.
All meta-analyses will be conducted using Stata version 12.0 (StataCorp LP, College Station, TX).
Grading the Evidence for Each Key Question
For key questions 1 and 3 we will grade asthma control composite scores, asthma specific hospitalizations, asthma-specific ED visits (asthma specific ICU admission/intubations) and asthma specific outpatient visits, asthma-specific detailed medication use, systemic corticosteroids for asthma, resource use, spirometry (FEV1 percent predicted) and quality of life. For key questions 2 and 4 we will grade harms: anaphylaxis, hypersensitivity adverse effects and death.
We will consider the strength of the study designs; randomized controlled trials will be graded as having the highest level of evidence. We will assess the quality and consistency of the available evidence, including assessment of the risk of bias in relevant studies, as well as aspects of directness, precision, and reporting bias as described in the Methods Guide for Effectiveness and Comparative Effectiveness Reviews38 and by Berkman and colleagues.39 Two reviewers will grade the pre-specified critical outcomes for each Key Question and then the entire team will discuss to reach consensus.
We will consider using GRADEpro to create summary tables.40
We will classify evidence pertaining to the Key Questions into four categories: (1) "High" grade (indicating high confidence that the evidence reflects the true effect and further research is very unlikely to change our confidence in the estimate of the effect); (2) "Moderate" grade (indicating moderate confidence that the evidence reflects the true effect but further research could change our confidence in the estimate of the effect and may change the estimate); (3) "Low" grade (indicating low confidence that the evidence reflects the true effect and further research is likely to change our confidence in the estimate of the effect and is likely to change the estimate); and (4) "Insufficient" grade (indicating evidence is unavailable or the body of evidence has unacceptable deficiencies, precluding reaching a conclusion).
Assessing Applicability
We will assess the applicability of studies in terms of the degree to which the study population, interventions, outcomes, and settings are typical for patients with asthma. Factors that may limit applicability include age, asthma severity or poorly controlled asthma, type of allergen used and dosage and use of cointervention or rescue medication.
The applicability of findings may also be limited by the types of asthma patients which are included in the study populations, as asthmatics with severe or poorly controlled asthma may be excluded in some controlled trials of immunotherapy. In addition, if studies do not clearly report immunotherapy dosing in units that can be translated to dosing with products available in the U.S., this may limit the applicability of recommendations of optimal dosing for U.S practitioners.
References
- National Heart, Lung, and Blood Institute. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Washington, DC: National Heart, Lung, and Blood Institute; 2007.
- National Heart, Lung, and Blood Institute. Advisory Council Asthma Expert Working Group. Needs Assessment Report for Potential Update of the Expert Panel Report-3 (2007): Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute; 2015. https://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/Asthma-Needs-Assessment-Report.pdf.
- Centers for Disease Control and Prevention. CDC. Most Recent Asthma Data. Atlanta, GA; 2014. http://www.cdc.gov/asthma/most_recent_data.htm July 25, 2016.
- American Lung Association. Trends in Asthma Morbidity and Mortality; 2012. http://www.lung.org/assets/documents/research/asthma-trend-report.pdf. Accessed on July 25, 2016.
- Global Asthma Network .GAN. The Global Asthma Report 2014. Auckland, New Zealand:; 2014. http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf.
- Centers for Disease Control and Prevention. CDC. FastStats; 2016. http://www.cdc.gov/nchs/fastats/asthma.htm. Accessed on April, 2016.
- Arbes SJ, Gergen PJ, Vaughn B, et al. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. Journal of Allergy and Clinical Immunology. 2007;120(5):1139-45.
- Noon L. Prophylactic Inoculation Against Hay Fever. The Lancet. 1911;177(4580):1572-3. doi: 10.1016/s0140-6736(00)78276-6.
- Sabin BR, Saltoun CA, Avila PC. Advances in upper airway diseases and allergen immunotherapy. J Allergy Clin Immunol. 2011 Feb;127(2):342-50. doi: 10.1016/j.jaci.2010.11.049. PMID: 21281864.
- Passalacqua G, Canonica GW. Allergen Immunotherapy: History and Future Developments. Immunol Allergy Clin North Am. 2016 Feb;36(1):1-12. doi: 10.1016/j.iac.2015.08.001. PMID: 26617223.
- Zimmer A, Bouley J, Le Mignon M, et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J Allergy Clin Immunol. 2012 Apr;129(4):1020-30. doi: 10.1016/j.jaci.2012.02.014. PMID: 22464673.
- Schulten V, Tripple V, Aasbjerg K, et al. Distinct modulation of allergic T cell responses by subcutaneous vs. sublingual allergen-specific immunotherapy. Clin Exp Allergy. 2016 Mar;46(3):439-48. doi: 10.1111/cea.12653. PMID: 26436865.
- Larenas-Linnemann D. Allergen immunotherapy: an update on protocols of administration. Curr Opin Allergy Clin Immunol. 2015 Dec;15(6):556-67. doi: 10.1097/aci.0000000000000220. PMID: 26485100.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011 Jan;127(1 Suppl):S1-55. doi: 10.1016/j.jaci.2010.09.034. PMID: 21122901.
- Hanci D, Sahin E, Muluk NB, et al. Immunotherapy in all aspects. Eur Arch Otorhinolaryngol. 2016 Jun;273(6):1347-55. doi: 10.1007/s00405-015-3553-5. PMID: 25673026.
- Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol Head Neck Surg. 2015 Feb;152(1 Suppl):S1-43. doi: 10.1177/0194599814561600. PMID: 25644617.
- Cox L, Larenas-Linnemann D, Lockey RF, et al. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010 Mar;125(3):569-74, 74.e1-74.e7. doi: 10.1016/j.jaci.2009.10.060. PMID: 20144472.
- Epstein TG, Liss GM, Murphy-Berendts K, et al. AAAAI and ACAAI surveillance study of subcutaneous immunotherapy, Year 3: what practices modify the risk of systemic reactions? Ann Allergy Asthma Immunol. 2013 Apr;110(4):274-8, 8.e1. doi: 10.1016/j.anai.2013.01.015. PMID: 23535092.
- Vaswani R, Garg A, Parikh L, et al. Non-adherence to subcutaneous allergen immunotherapy: inadequate health insurance coverage is the leading cause. Annals of Allergy, Asthma & Immunology. 2015;115(3):241-3.
- Broide DH. Immunomodulation of allergic disease. Annu Rev Med. 2009;60:279-91. doi: 10.1146/annurev.med.60.041807.123524. PMID: 19630573.
- Focke M, Swoboda I, Marth K, et al. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010 Mar;40(3):385-97. doi: 10.1111/j.1365-2222.2009.03443.x. PMID: 20210812.
- Valenta R, Campana R, Focke-Tejkl M, et al. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016 Feb;137(2):351-7. doi: 10.1016/j.jaci.2015.12.1299. PMID: 26853127.
- Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016 Jun;24(3):215-20. doi: 10.1097/moo.0000000000000249. PMID: 27159540.
- Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010(8):Cd001186. doi: 10.1002/14651858.CD001186.pub2. PMID: 20687065.
- Cox L, Esch RE, Corbett M, et al. Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes. Ann Allergy Asthma Immunol. 2011 Oct;107(4):289-99; quiz 300. doi: 10.1016/j.anai.2011.06.018. PMID: 21962088.
- Lin SY, Erekosima N, Suarez-Cuervo C, et al. AHRQ Comparative Effectiveness Reviews. Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013.
- Hurst DS, Gordon BR, Fornadley JA, et al. Safety of home-based and office allergy immunotherapy: a multicenter prospective study. Otolaryngology-Head and Neck Surgery. 1999;121(5):553-61.
- FDA. Food and Drug Administration. Statistical Review - GRASTEK2014 2013. http://www.fda.gov/downloads/BiologicsBloodVaccines/Allergenics/UCM394338.pdf. Accessed on May 20, 2016.
- FDA. Food and Drug Administration. Statistical Review - ORALAIR2014 2012. http://www.fda.gov/downloads/BiologicsBloodVaccines/Allergenics/UCM393025.pdf. Accessed on May 20, 2016.
- FDA. Food and Drug Administration. Statistical Review Memo- RAGWITEK2014.; 2013. http://www.fda.gov/downloads/BiologicsBloodVaccines/Allergenics/UCM397307.pdf. Accessed on May 20, 2016.
- FDA. Allergen Extract Sublingual Tablets. Silver Spring MD: U.S. Food and Drug Administration; 2014. http://www.fda.gov/BiologicsBloodVaccines/Allergenics/ucm391505.htm. Accessed on May 20, 2014.
- American Academy of Allergy Asthma & Immunology. Allergic Asthma. Milwaukee, WI; 2015. http://www.aaaai.org/conditions-and-treatments/conditions-dictionary/allergic-asthma. Accessed on May, 2016.
- Busse WW, Morgan WJ, Taggart V, et al. Asthma outcomes workshop: overview. Journal of Allergy and Clinical Immunology. 2012;129(3):S1-S8.
- Higgins J, Green S. Cochrane handbook for systematic reviews London, England: The Cochrane Collaboration; 2011. www.cochrane-handbook.orgVersion 5.1.0.
- Sterne J, Higgins J, Reeves B. on behalf of the development group for ROBINS-I: a tool for assessing Risk Of Bias In Non-randomized Studies of Interventions, Version 5 July 2016. 2014. http://www.riskofbias.info Accessed on August 3, 2016.
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. The Lancet. 2000 10/7/;356(9237):1255-9. doi: http://dx.doi.org/10.1016/S0140-6736(00)02799-9.
- Cornell JE, Mulrow CD, Localio R, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Annals of internal medicine. 2014;160(4):267-70.
- Agency for Healthcare Research and Quality. AHRQ. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. . Rockville, MD: Agency for Healthcare Research and Quality; 2014. Chapters available at: www.effectivehealthcare.ahrq.gov Accessed on May 15, 2016.
- Berkman ND, Lohr KN, Ansari MT, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol. 2015 Nov;68(11):1312-24. doi: 10.1016/j.jclinepi.2014.11.023. PMID: 25721570.
- GRADEpro. GRADE's software for Summary of Findings tables, Health Technology Assessment and Guidelines. 2015. https://gradepro.org/. Accessed on August 22, 2016.
Definition of Terms
Definitions are listed in Appendix 1.
Summary of Protocol Amendments
In the event of protocol amendments, the date of each amendment will be accompanied by a description of the change and the rationale.
Review of Key Questions
AHRQ posted the key questions on the Effective Health Care Website for public comment. The EPC refined and finalized the key questions after review of the public comments, and input from Key Informants and the Technical Expert Panel (TEP). This input is intended to ensure that the key questions are specific and relevant.
Technical Expert Panel (TEP)
We will select a TEP to provide broad expertise and perspectives specific to the topic under development. The TEP will not perform analysis of any kind nor contribute to the writing of the report.
Peer Review
Approximately five experts in the field will be asked to peer review the draft report and provide comments. The peer reviewers may represent stakeholder groups such as professional or advocacy organizations with knowledge of the topic.
Appendix
Appendix 1 - List of definitions
1. Immunotherapy Outcome Terms
- Allergic asthma remission: capability of specific immunotherapy (AIT) to result in remission of the disease itself; this may be manifest as an effect of continued treatment or may be observed even once AIT is discontinued.
- Disease modification: capability of AIT to modify the clinical course and natural history of asthma and attenuate disease symptomatology and underlying pathophysiology.
- Modulation of immune system: capability of AIT to modify the immunologic pathways that are responsible for, or play a role in, the disease process.
- Immune tolerance: capability of AIT to induce tolerance with suppression of the untoward clinical and immunologic response.
- Maintenance control: capability of AIT to provide sustained clinical benefit with continued use.
2. Mechanistic Terms
- Immunoglobulins (Ig): Multi-subunit proteins which function in IMMUNITY. They are produced by B lymphocytes from the Immunoglobulins genes. They are comprised of two heavy chains (Immunoglobulins heavy chains) and two light chains (Immunoglobulins light chains) with additional ancillary polypeptide chains depending on their isoforms. The variety of isoforms includes monomeric or polymeric forms, and transmembrane forms (B-Cell antigen receptors) or secreted forms (antibodies). They are divided by the amino acid sequence of their heavy chains into five classes; Immunoglobulin A (IgA), Immunoglobulin D (IgD), Immunoglobulins E (IgE), Immunoglobulin G (IgG), Immunoglobulin M (IgM), and various subclasses.
- IgG: The major immunoglobulin isotype class in normal human serum. There are several isotype subclasses of IgG, for example, IgG1, IgG4, IgG2A, IgG2B. (MeSH)
- IgE: An immunoglobulin associated with mast cells. Overexpression has been associated with allergic hypersensitivity (HYPERSENSITIVITY, IMMEDIATE). (MeSH)
- All other immunologic parameters, such as T-Lymphocytes (Lymphocytes responsible for cell-mediated immunity), cytokines (IL4/IL5/IL10/etc, non-antibody proteins that act as intercellular mediators) will not be included as outcomes.
3. Objective Tests
- Spirometry (FEV1;FVC;FEV1/FVC ratio)
- [peak expiratory flow rate]: as opposed to formal spirometry (which is performed in a physician's office), the patient can use a home peak flow meter (hand-held device) to check his/her peak flow readings on a regular basis. These measurements are simple and easy to do and provide the patient with immediate feedback with which to monitor his asthma and make medication adjustments on a daily basis.
- Allergen challenge testing: research tool in which allergen is introduced into the airways in a controlled fashion in order to reproduce allergen-induced asthma symptoms and characterize the patient's allergic response and response to treatment.
- Methacholine challenge: research tool in which a chemical irritant substance is inhaled into the airways in a controlled fashion in order to induce asthma symptoms. It can be used to diagnose asthma, characterize the severity of asthma, and/or assess the patient's response to treatment.
4. Medications
Definition of medication purpose
- Long-term controller medications: They should be taken daily to achieve and maintain control of persistent asthma. They work over time rather than immediately, reduce or prevent inflammation and scarring of airways. (Include inhaled corticosteroids, cromolyn, immunomodulatory, leukotriene modifiers, methylaxanthines, long-acting bronchodilators.)
- Quick relief medication: Rescue/reliever medications are fast-actingmedications used to relieve asthma symptoms when they occur. These types of medicines are often inhaled directly into the lungs, where they open up the airways and relieve symptoms such as wheezing, coughing, and shortness of breath. But as effective as they are, rescue medications don't have a long-term effect. (Include anticholinergics, SABAs (albuterol, levalbuterol) and systemic corticosteroids)
- Placebo: Any dummy medication or treatment. Although placebos originally were medicinal preparations having no specific pharmacological activity against a targeted condition, the concept has been extended to include treatments or procedures, especially those administered to control groups in clinical trials in order to provide baseline measurements for the experimental protocol.
Medications for asthma care per EPR3 recommendations
- Corticosteroids
- Inhaled corticosteroid (ICS): beclomethasone dipropionate (QVAR, Vanceril, Beclovent)), budesonide (Pulmicort), flunisolide (Aerobid), fluticasone propionate (Flovent), triamcinolone acetonide (Azmacort)
- Leukotriene antagonist (LTRa): A class of drugs designed to prevent leukotriene synthesis or activity by blocking binding at the receptor level. (MeSH)
- montelukast (Singulair), zafirlukast (Accolate), zileuton (Zyflo)
- Antihistamine (Histamine H1 antagonists): Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (MeSH)
- brompheniramine (Dimetapp), chlorpheniramine (Chlor-Trimeton), dimenhydrinate (Dramamine), diphenhydramine (Benadryl), doxylamine (Vicks NyQuil), loratadine (Alavert, Claritin), cetirizine (Zyrtec)
- Cromolyn (Cromolyn sodium): A chromone complex that acts by inhibiting the release of chemical mediators from sensitized mast cells. It is used in the prophylactic treatment of both allergic and exercise-induced asthma, but does not affect an established asthmatic attack. (MeSH)
5. Efficacy measures
- Asthma symptom diaries: Recorded self-assessment of asthma symptoms.
- Asthma Medication use: Need of daily medications. Reduction in long-term controller medication and quick relief medication.
- Quality of life (QOL): Asthma Quality of Life Questionnaire: There are 32 questions in the AQLQ and they are in 4 domains (symptoms, activity limitation, emotional function and environmental stimuli). The activity domain contains 5 'patient-specific' questions. This allows patients to select 5 activities in which they are most limited and these activities will be assessed at each follow-up. Patients are asked to think about how they have been during the previous two weeks and to respond to each of the 32 questions on a 7-point scale (7 = not impaired at all - 1 = severely impaired). The overall AQLQ score is the mean of all 32 responses and the individual domain scores are the means of the items in those domains. (Includes strenuous activities (such as hurrying, exercising, running upstairs, sports), moderate activities (such as walking, housework, gardening, shopping, climbing stairs), social activities (such as talking, playing with pets/children, visiting friends/relatives), work-related activities, and sleeping.
6. Safety terms
- Adverse events (AE): An injury caused by medical management–rather than by the underlying disease–which prolongs hospitalization, produces a disability at the time of discharge, or both. Etiology: Drug effects, wound infections, technical complications, negligence, diagnostic mishaps, therapeutic mishaps, and events occurring in the emergency room. (McGraw Hill Concise Dictionary of Modern Medicine, 2002)
- An adverse event is any undesirable experience associated with the use of a medical product in a patient. (Food and Drug Administration, 2009: http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.htm )
- Serious adverse events (SAE): The event is serious and should be reported when the patient outcome is: death, life-threatening, hospitalization (initial or prolonged), disability, congenital anomaly, or requires intervention to prevent permanent impairment or damage. (Food and Drug Administration, 2009)
- When a particular condition causes the immune system to overreact, it is referred to as hypersensitivity reaction that triggers the production of IgGE. These reactions may be damaging, uncomfortable, or occasionally fatal. https://www.aaaai.org/conditions-and-treatments/conditions-dictionary/hypersensitivity-reactions
- Anaphylaxis: An acute hypersensitivity reaction (Type I IgE mediated allergic immediate reaction) due to exposure to a previously encountered ANTIGEN. The reaction may include rapidly progressing URTICARIA, respiratory distress, vascular collapse, systemic SHOCK, and death. (MeSH)






















.png)









No hay comentarios:
Publicar un comentario